Adhesive based therapy for varicose veins approved by FDA
- The US FDA (Food and Drug Administration) on 20th February 2015, approved a novel adhesive based therapy for the treatment of varicose veins.
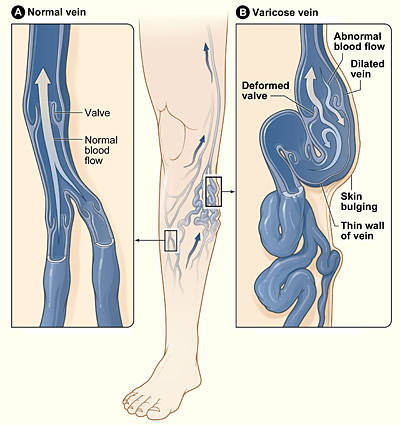
- VenaSeal system produced by Covidien LLC is used for the treatment of symptomatic superficial varicose veins in the lower limbs.
- The adhesive used is n-butyl-2-cyanoacrylate.
- The adhesive delivery system consists of a catheter, guidewire, dispenser gun, dispenser tips and syringes.
- The catheter is inserted via ultrasound guidance into the varicose vein.
- The adhesive is introduced as a clear liquid which polymerises into a solid.
- It was found to be as safe and effective as radiofrequency ablation in a manufacturer sponsored clinical trial .
Advantages
- Does not involve application of heat / surgery.
- It allows faster return to normal daily routine.
Contraindications
- Hypersensitivity to the adhesive.
- Acute phlebitis due to blood clots.
- Whole body acute infection.
Adverse effects
- Phlebitis
- Paresthesia
Source : http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm435082.htm